The lawsuit was filed by Mel L., a man who was implanted with the C.R. Bard Meridian® Vena Cava Filter on October 29, 2012 at a hospital in Missouri.
Meridian is implanted in a major blood vessel called the inferior vena cava (IVC) to catch blood clots. It prevents clots from reaching the lungs and causing a deadly pulmonary embolism.
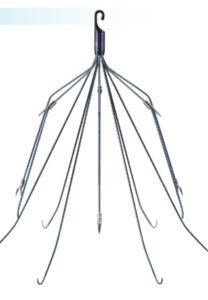
C.R. Bard Meridian® IVC Filter
Meridian was introduced in 2011 without clinical trials because it was “equivalent” to other IVC filters made by C.R. Bard. Like these other filters, Meridian was soon pulled off the market without a recall.
The “equivalent” filters include the Bard G2 and Recovery, which have been associated with hundreds of injuries, dozens of deaths, and high rates of fracture with embolization of broken fragments to vital organs.
The needle-like wire legs can also puncture through the wall of the blood vessel or become embedded. The risk of fracture, tilting, migration, and perforation increases the longer Meridian is implanted.
Lawyers accuse C.R. Bard of selling a defective and unreasonably dangerous medical device, failing to warn about safety risks, and inadequately testing Meridian for safety before marketing it as “safe.”
The lawsuit was filed on December 20, 2016 in the U.S. District Court for the District of Arizona — Case No. 2:16-cv-04486.
It will be centralized with over 1,283 other IVC filter lawsuits now pending against C.R. Bard in Multi-District Litigation (MDL No. 2641)— In Re: Bard IVC Filters Products Liability Litigation.
The plaintiff is represented by Ben C. Martin of The Law Offices of Ben C. Martin in Dallas, Texas. He serves on the plaintiffs’ steering committee of the Bard IVC Filter MDL.
 Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
Editor’s note: For more information about IVC Filter lawsuits and your legal rights, please contact The Law Offices of Ben C. Martin.
